Diagnostics
for Lung Infections
Pearl Diagnostics is changing the way clinicians diagnose lung infections. Our in vitro diagnostics identify circulating microbial extracellular vesicles.
Why Extracellular Vesicles?
Extracellular vesicles (EVs) are made by every cell as a means to communicate. Pearl Diagnostics has discovered that microbial EVs can be detected in urine as an early indicator of lung infections.
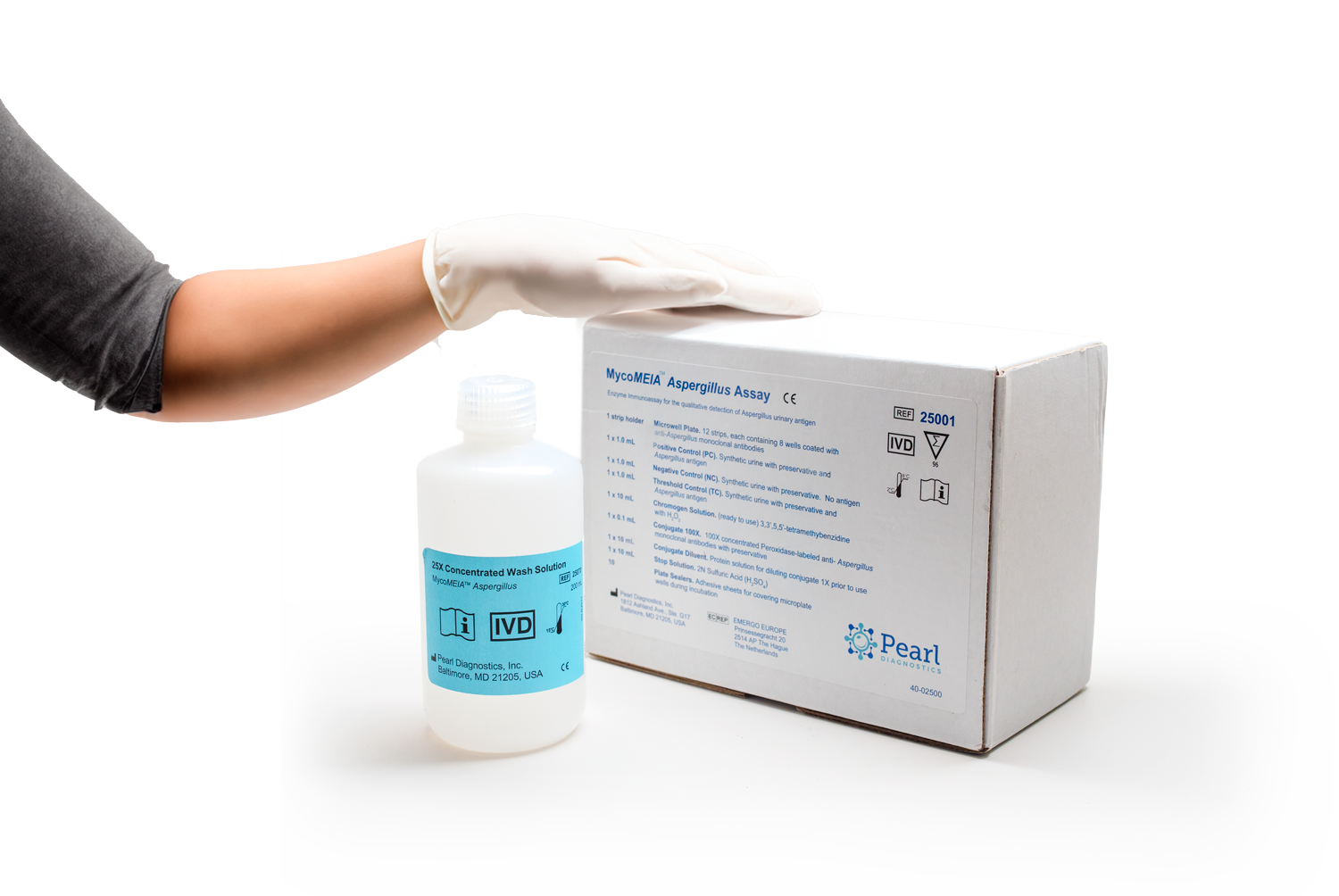
Our Products*
Learn more about Pearl Diagnostic’s novel in vitro diagnostic assays for lung infections.
*Not available in the US





